In silico engineering of ion-exchanged zeolites for high-performance carbon capture in PSA processes
Abstract
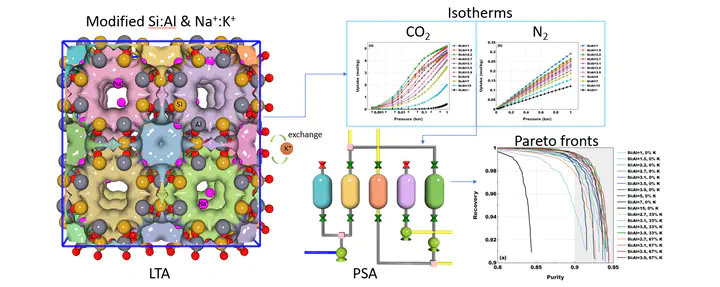
In this article we employ a multiscale computational framework to design zeolites with competitive performance in post-combustion carbon capture. The efficiency of CO2 capture from flue gas critically depends on the adsorption properties of the adsorbent material. In recent studies, the search for the best performing materials has been predominantly confined to Metal-Organic Frameworks (MOFs), due to the wide diversity in their adsorption characteristics. Inspired by MOFs and their properties, we sought to produce a similar variety in the CO2 isotherms of zeolites. To this end, we modified the properties of the well-known LTA zeolite by varying the Si:Al ratio and the cation composition. Using molecular simulations, we show that these in silico engineered zeolites exhibit a wide range of isotherms for carbon capture. Pressure Swing Adsorption (PSA) simulations using the modified Skarstrom cycle show that structures with the Si:Al ratio ranging from 2.2 to 3.9 can meet the standard requirement of 90 % purity at 90 % recovery for CO2. This performance can be further improved by modifying the cation composition via moderate ion-exchange of Na+ with K+ ions. Several designed structures exhibit performance that is comparable to or even better than the current industrial benchmark, Zeolite l3X, therefore offering interesting alternatives to MOFs as adsorbents for carbon capture in PSA.